Introduction to CBD
Cannabidiol, or CBD, is one of the two most well-known compounds derived from the cannabis plant, with the other one being THC. CBD is the non-intoxicating cousin of THC, meaning that it does not make you feel ‘high’ like THC does. Although sometimes mistakenly called ‘non-psychoactive’, it actually can affect the mind, as we will discuss on this page.
Where does CBD come from?
Technically, the cannabis plant does not produce THC and CBD, but THCA and CBDA – we explained it in the
Introduction to THC chapter. CBDA, or CBD acid, is produced by the plant from the parent compound CBGA, another cannabinoid.
1 Once formed, CBDA is only converted to CBD through a biochemical reaction called
decarboxylation, as demonstrated in Figure 1.
2, 3 Typically, this process happens by heat. CBDA and CBD are both pharmacologically active in the body, but their properties differ significantly. It should be noted that many CBD products that list CBD on their content labels reflect the sum of CBD + CBDA instead of CBD alone.
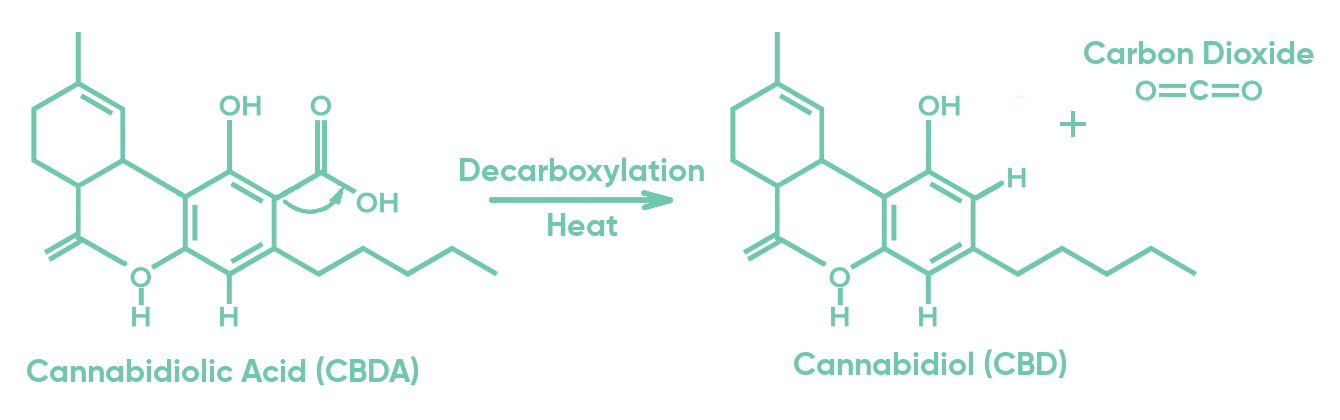 Figure 1: The transformation of CBDA molecule into CBD + CO2 by heat.
Is CBD in hemp and marijuana different?
Figure 1: The transformation of CBDA molecule into CBD + CO2 by heat.
Is CBD in hemp and marijuana different?
Hemp and marijuana are different ways of referring to the same cannabis plant, as explained in the
Marijuana vs. hemp chapter. CBD is produced by cannabis, and it doesn’t matter if it’s produced by the hemp-type (industrial) cannabis, or by the drug-type cannabis (‘marijuana’). Some people want to know whether CBD from marijuana is different than CBD from hemp. Although there can be different quantities of various cannabinoids and other compounds in hemp and marijuana, the CBD compound is the same in either plant.
The therapeutic effects of CBD
CBD is actively being studied in various clinical trials, so its effectiveness in various conditions is still being established. For now, what we know with certainty is that CBD has two main therapeutic effects: decreasing anxiety
4 and decreasing epileptic seizures in some forms of epilepsy. The latter was recognized by regulatory bodies, which resulted in a plant-derived extract with a high CBD content that was FDA-approved in 2018 for the treatment of Lennox-Gastaut Syndrome and Dravet Syndrome, rare types of epilepsy in children. The CBD product is called Epidiolex in the United States and Epidyolex in Europe.
5 Although cannabis compounds such as CBD are controlled substances and fall under schedule 1, which is the most strict category in the US, when sold as Epidiolex, CBD is not considered a controlled substance.
6
Various studies have found that CBD helps against anxiety.
7 The studies have mainly been done with only one dose of CBD and also in very limited patient groups, but at least two studies are underway to understand more about the types of anxiety, as well as the effects when CBD is not taken once, but over a longer period of time.
8 If you want to learn more about the effects of CBD on anxiety, take a look at our section on
Anxiety.
Nabiximols
CBD is also a part of THC+CBD medication nabiximols, sold as Sativex, which is used for the treatment of spasticity in patients suffering from multiple sclerosis.
9 Although Sativex is sold in almost 30 countries, the medicine is not approved in the United States. We will talk more about THC and CBD interaction in one of the upcoming sections.
Preliminary results
Animal studies and preliminary clinical trials have demonstrated effects of CBD in additional types of epilepsy, certain types of chronic pain, various types of anxiety, psychosis and schizophrenia, and substance abuse.
8, 10 Although encouraging, these results may not be translatable to patients. We need to keep in mind that treating animals and humans, or healthy people and patients, differs greatly. It is also important to note that one successful study does not mean that the compound is effective as a medicine, so more research is necessary to support it. Luckily, there are ongoing studies that should determine the effectiveness of different kinds of CBD products on different conditions.
False claims
In various places on the internet and in shops, you will see that all sorts of claims are attributed to CBD, which have not necessarily been verified by science. Sometimes, that is because people report their personal experiences, and other times, the claims can be interpretations of science that was done in animal studies or in preliminary clinical trials. Because those claims have not been scientifically or medically verified, and can be inaccurate, the FDA has been known to send warning letters to companies and other organizations for doing so. A well-known example is the claim that CBD can cure COVID-19 that was made by various CBD companies.
11 It is important to verify any medical claim with your physician.
The interaction of CBD and THC
Most of the medicinal workings of cannabis that we know
come from THC. Next to its medicinal workings, THC is generally not well tolerated due to its intoxicating side-effects. CBD can decrease some, but not all of these side-effects. The side-effects that can be decreased include anxiety, psychotic-like effects, and cognitive effects, such as memory impairment.
12, 13, 14
Various products, such as the pharmaceutical product nabiximols (Sativex), contain THC+CBD. Although some people believe that CBD and THC improve each other’s workings, e.g. by giving a stronger effect on pain relief, scientific studies in humans have shown mixed results.
How does CBD work in the body?
In contrast to THC, which exerts most of its effects via just one receptor, the effects by CBD are the result of binding to many different
receptors and other proteins. This underlies its diverse effects in different conditions. Although many sources try to make people believe that CBD directly works on your
endocannabinoid system (ECS), the role of CB
1 and CB2 are generally negligible.
15
CBD and the ECS
CBD does not directly activate the CB
1 receptor, which is the reason for its non-intoxicating properties. Laboratory studies show that CBD binds to a different site on the CB
1 receptor and not on the main site, where THC binds. CBD binds to a site where it can only reduce receptor activation when another compound is bound to the main site. This makes CBD a so-called negative allosteric modulator of CB
1. In theory, this can make CBD influence THC’s effects of ‘Feeling High’, but in practice, the CBD dose is often not high enough to make this happen.
14
From laboratory studies, it was also found that CBD can raise levels of the endocannabinoid AEA (anandamide), which activates the CB
1 receptor. The mechanism in humans is believed to work by CBD inhibiting the transporters in the cell, whereby AEA is not brought to the enzymes that break them down. In rats, a different mechanism of CBD was found in which cannabidiol inhibits the AEA break-down enzyme FAAH.
16 This action is an ‘opposing’ effect to its downregulation of CB
1 activation.
CBD outside of the ECS
CBD has many pharmacological effects beyond direct interactions with the ECS. In this section we will discuss a few, but not all of them may contribute to the therapeutic effects of CBD. It is likely that CBD’s effects are induced by a combination of different actions, and not by just one action alone.
Firstly, CBD is an agonist of the serotonin receptor 5-HT1A. This receptor plays an important role in anxiety, and via this interaction, CBD induces its anxiolytic (anti-anxiety) effects.
17 The counteracting effects of CBD on THC-induced anxiety, is also believed to work via the serotonin system.
CBD also works on the dopamine system. Dopamine is involved in happiness and in psychosis. CBD is a partial agonist of a particular dopamine D2 receptor.
18 Via this interaction, CBD is believed to decrease (THC-induced) psychotic effects.
You might have heard of TRPV1 before: this
ion channel is well-known for capsaicin binding and making you feel the spiciness of hot peppers.
19 CBD also binds on the TRPV1 channel, but eventually desensitizes it, thereby contributing to its pain relieving (analgesic) effects.
20, 21
Other receptors that CBD binds to include TRPV2, PPARγ and adenosine receptors.
22, 23
The side effects of CBD
Compared to THC, CBD is very well tolerated. CBD has been given to people in a single dose of 6 grams. However, CBD can give side effects, such as nausea, vomiting and sleep problems, but most patients tolerate single doses of CBD well with no intolerable side effects.
5
Side effects after long-term use are still being studied. Most patients tolerate long-term CBD with no major issues. Some animal and human studies have identified liver-related risks, such as elevated transaminases. For this reason, liver enzymes may be monitored during chronic CBD treatment.
5
An additional risk of CBD is
drug-drug interactions. CBD can
inhibit specific metabolic enzymes, called cytochrome P450 (CYP450), most notably type CYP2C19.
5 Patients taking additional medications should check with a pharmacist about possible interactions with CBD.
CBD has been studied relatively extensively in patients with epilepsy. These patients typically use various medications. It is possible that some CBD side effects are a result of drug-drug interactions of CBD and other medications that are given around the same time. This interaction could lead to an increase of the concentration of the other medication in the body, thereby causing side effects. It is therefore possible that the side effects of CBD are actually caused by other medications, but further studies need to examine this better.
- Pertwee, Roger G. (2014). Handbook of Cannabis. Handbooks in Psychopharmacology, 44(8), 085201.
- Shoyama, Yukihiro; Yagi, Masahiro; Nishioka, Itsuo; Yamauchi, Tatsuo (1975). Biosynthesis of cannabinoid acids. Phytochemistry, 14(10), 2189-2192.
- Taura, Futoshi; Sirikantaramas, Supaart; Shoyama, Yoshinari; Yoshikai, Kazuyoshi; Shoyama, Yukihiro; Morimoto, Satoshi (2007). Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Letters, 581(16), 2929-2934.
- Bergamaschi, M. M.; Queiroz, R. H. C.; Chagas, M. H. N.; de Oliveira, D. C. G.; De Martinis, B. S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, Antonio E.; Martín-Santos, R.; Hallak, J. E. C.; Zuardi, A. W.; Crippa, J. A. S. (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology, 36(6), 1219--26.
- U. S. Food and Drug Administration (2018). Product label of Epidiolex.
- GW Pharmaceuticals. (2020). GW Pharmaceuticals plc and Its U.S. Subsidiary Greenwich Biosciences, Inc. Announce That EPIDIOLEX® (cannabidiol) Oral Solution Has Been Descheduled And Is No Longer A Controlled Substance.
- Zuardi, A. W.; Cosme, R. A.; Graeff, F. G.; Guimaraes, F. S. (1993). Effects of ipsapirone and cannabidiol on human experimental anxiety. Journal of Psychopharmacology, 7(1suppl), 82--88.
- NIH, U.S. National Library of Medicine (2020). 230 studies on cannabidiol.
- Electronic Medicines Compendium (2019). Summary of Product Characteristics (SmPC) of Sativex, version 03.
- Hindocha, Chandni; Freeman, Tom P.; Grabski, Meryem; Stroud, Jack B.; Crudgington, Holly; Davies, Alan C.; Das, Ravi K.; Lawn, William; Morgan, Celia J. A.; Curran, H. Valerie (2018). Cannabidiol reverses attentional bias to cigarette cues in a human experimental model oftobacco withdrawal. Addiction, 113(9), 1696-1705.
- U. S. Food and Drug Administration (2020). Warning letter to Nova Botanix LTD DBA CanaBD. U.S. Food and Drug Administration.
- Karniol, I. G.; Shirakawa, I.; Kasinski, N.; Pfeferman, A.; Carlini, E. A. (1974). Cannabidiol interferes with the effects of delta 9 - tetrahydrocannabinol in man. European journal of pharmacology, 28(1), 172--177.
- Zuardi, A. W.; Shirakawa, I.; Finkelfarb, E.; Karniol, I. G. (1982). Action of cannabidiol on the anxiety and other effects produced by ?9-THC in normal subjects. Psychopharmacology, 76(3), 245--250.
- Freeman, Abigail M.; Petrilli, Katherine; Lees, Rachel; Hindocha, Chandni; Mokrysz, Claire; Curran, H. Valerie; Saunders, Rob; Freeman, Tom P. (2019). How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neuroscience & Biobehavioral Reviews, 107, 696-712.
- Fernández-Ruiz, Javier; Sagredo, Onintza; Pazos, M. Ruth; García, Concepción; Pertwee, Roger; Mechoulam, Raphael; Martínez-Orgado, José (2013). Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid?. British Journal of Clinical Pharmacology, 75(2), 323-333.
- Elmes, Matthew W.; Kaczocha, Martin; Berger, William T.; Leung, KwanNok; Ralph, Brian P.; Wang, Liqun; Sweeney, Joseph M.; Miyauchi, Jeremy T.; Tsirka, Stella E.; Ojima, Iwao; Deutsch, Dale G. (2015). Fatty Acid-binding Proteins (FABPs) Are Intracellular Carriers for Δ 9 -Tetrahydrocannabinol (THC) and Cannabidiol (CBD). Journal of Biological Chemistry, 290(14), 8711-8721.
- Gomes, Felipe V.; Resstel, Leonardo B. M.; Guimarães, Francisco S. (2011). The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology, 213(2-3), 465-473.
- Seeman, P (2016). Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Translational Psychiatry, 6(10), e920-e920.
- Frias, Bárbara; Merighi, Adalberto (2016). Capsaicin, Nociception and Pain. Molecules, 21(6), 797.
- Bisogno, Tiziana Hanuš,; Lumír De Petrocellis, Luciano; Tchilibon, Susanna; Ponde, Datta E.; Brandi, Ines; Moriello, Aniello Schiano; Davis, John B.; Mechoulam, Raphael; Di Marzo, Vincenzo (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology, 134(4), 845-852.
- Iannotti, Fabio Arturo; Hill, Charlotte L.; Leo, Antonio; Alhusaini, Ahlam; Soubrane, Camille; Mazzarella, Enrico; Russo, Emilio; Whalley, Benjamin J.; Di Marzo, Vincenzo; Stephens, Gary J. (2014). Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chemical Neuroscience, 5(11), 1131-1141.
- De Petrocellis, Luciano; Ligresti, Alessia; Moriello, Aniello Schiano; Allarà, Marco; Bisogno, Tiziana; Petrosino, Stefania; Stott, Colin G.; Di Marzo, Vincenzo (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology, 163(7), 1479--1494.
- Pertwee, R. G. (2008). The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9 -tetrahydrocannabinol, cannabidiol and Δ 9 -tetrahydrocannabivarin. British Journal of Pharmacology, 2(199-215)
 Figure 1: The transformation of CBDA molecule into CBD + CO2 by heat.
Is CBD in hemp and marijuana different?
Hemp and marijuana are different ways of referring to the same cannabis plant, as explained in the Marijuana vs. hemp chapter. CBD is produced by cannabis, and it doesn’t matter if it’s produced by the hemp-type (industrial) cannabis, or by the drug-type cannabis (‘marijuana’). Some people want to know whether CBD from marijuana is different than CBD from hemp. Although there can be different quantities of various cannabinoids and other compounds in hemp and marijuana, the CBD compound is the same in either plant.
Figure 1: The transformation of CBDA molecule into CBD + CO2 by heat.
Is CBD in hemp and marijuana different?
Hemp and marijuana are different ways of referring to the same cannabis plant, as explained in the Marijuana vs. hemp chapter. CBD is produced by cannabis, and it doesn’t matter if it’s produced by the hemp-type (industrial) cannabis, or by the drug-type cannabis (‘marijuana’). Some people want to know whether CBD from marijuana is different than CBD from hemp. Although there can be different quantities of various cannabinoids and other compounds in hemp and marijuana, the CBD compound is the same in either plant.
_logo.svg)