Hydrocarbon extraction
Hydrocarbon extraction is one of the various ways to separate
cannabinoids and terpenes from other plant material. The solvents used for this extraction are hydrocarbons, which are compounds made of only hydrogen and carbon atoms. The most commonly used hydrocarbons for extraction are
butane and
propane. The benefit of these two solvents is that their boiling point is relatively low: at room temperature, butane and propane are gasses.
1 This means that the solvents easily evaporate and leave behind the extract or concentrate, thereby minimizing the risk of ingesting the toxic solvents that stay behind, also referred to as
residual solvents.
What makes hydrocarbon extraction different?
Hydrocarbons are non-polar, which means that they are particularly good at separating terpenes, compared to other solvent-based extraction techniques. In Figure 1, you can see the difference between
hemp flower prior to and after hydrocarbon extraction. Prior to extraction, flower contains approximately 21% cannabinoids and 2% terpenes, measured by weight. This means that there are approximately 10 times more cannabinoids than terpenes in cannabis flower. When looking at what happens after the extraction, the relative cannabinoid contents make up 80% and the terpene contents 20%. Now, there are about 4 times more cannabinoid than terpene contents. This means that during extraction, the yield of terpenes is relatively higher than the cannabinoid yield.
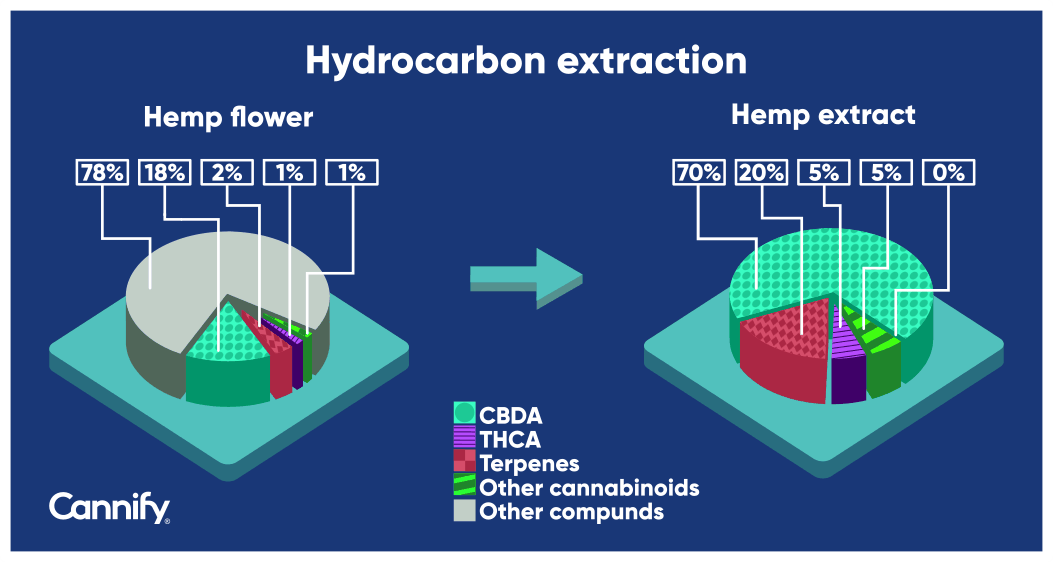 Figure 1: The difference between the contents of cannabis compounds in a raw hemp flower and a hydrocarbon hemp extract (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/)
Figure 1: The difference between the contents of cannabis compounds in a raw hemp flower and a hydrocarbon hemp extract (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/)
BHO and PHO
BHO stands for
butane hash oil and is sometimes also called butane honey oil due to its honey-like appearance (Figure 2). BHO refers to the extract produced after butane extraction, whereas the less commonly used abbreviation PHO, refers to the product after propane extraction. Due to its high concentration of pharmacologically active compounds compared to cannabis flower, hash oils give stronger effects than flower. The oil is typically used for inhalation after heating, but it is also used orally in edibles. Further processing of BHO and PHO will lead to a variety of products, whose names are related to their appearance, such as wax and shatter.
 Figure 2: Butane bubbles evaporating before whipping and vacuum purging (image taken from Vjiced, Butane honey oil being evaporated, CC BY-SA 3.0).
Figure 2: Butane bubbles evaporating before whipping and vacuum purging (image taken from Vjiced, Butane honey oil being evaporated, CC BY-SA 3.0).
Residual solvents
Although correct purging (see the paragraph about the steps of extraction) of the solvents from the hydrocarbon extract should not leave any residuals behind, purging does not always happen correctly or fully. A 2015 study described that analysis of various extracts found various residual solvents, including butane.
1 More than 80% of the products analyzed contained residual solvents. Many jurisdictions require testing for the presence and quantity of residual solvents. Customers should be able to get a copy of the certificate of analysis for residual solvents from the manufacturer.
Hydrocarbon extraction has some negative connotation as it can be a dangerous business: hydrocarbon extraction requires the right skills and safety precautions. Although safe closed-loop systems {call-out, text:A system in which the output is used as the input\, and there is no contact with the outside world.} are available and even required in various jurisdictions, outside of the legal or professional world, hydrocarbon extraction is often performed by non-trained enthusiasts in garages, tool sheds, and vacant homes.
2 Due to the high pressure under which the hydrocarbons are kept, failing actions, processes, or materials can lead to dangerous situations, such as explosions.
3, 4 Also, when not leading to an explosion, hydrocarbon purging {call-out, text:Removing gaseous solvents from the extract.} and subsequent inhalation of the toxic hydrocarbons can potentially result in heart or brain damage.
5, 6
The process begins with the extractor {call-out, text:A person performing the extraction procedure.} filling a stainless-steel column with ground plant material which is then hooked up to a tank (Figure 3) filled with a hydrocarbon liquid. Because hydrocarbons are gases at room temperature, they are pressurized and/or cooled to bring them to a liquid state. Once the hydrocarbons wash over the plant material in the column, terpenes and cannabinoids are dissolved. The mixture flows down the column by gravity into a collection chamber at the bottom of the extraction column (Figure 4). Once the wash is complete, the valves for the hydrocarbon and the column are closed. Then the collection chamber is detached from the extraction column and the mixture is poured onto a dish, commonly a borosilicate (heat-resistant) glass tray. The tray is placed inside of a temperature-controlled vacuum oven to begin pulling the vacuum on the mixture. This lowers the boiling point {call-out, text:The temperature at which a liquid changes into a gas.} of any compounds in the mixture, thereby removing the hydrocarbons from the extract. This process is often referred to as ‘purging.’
1, 7
Once the purging is complete, the pressure inside the oven is brought back to normal atmospheric pressure, after which the oven can be opened and the extract taken out for further refinement or packaging.
 Figure 3: MK III Closed loop essential oil extractor (image taken from Emerald Gold Extractors).
Figure 3: MK III Closed loop essential oil extractor (image taken from Emerald Gold Extractors).
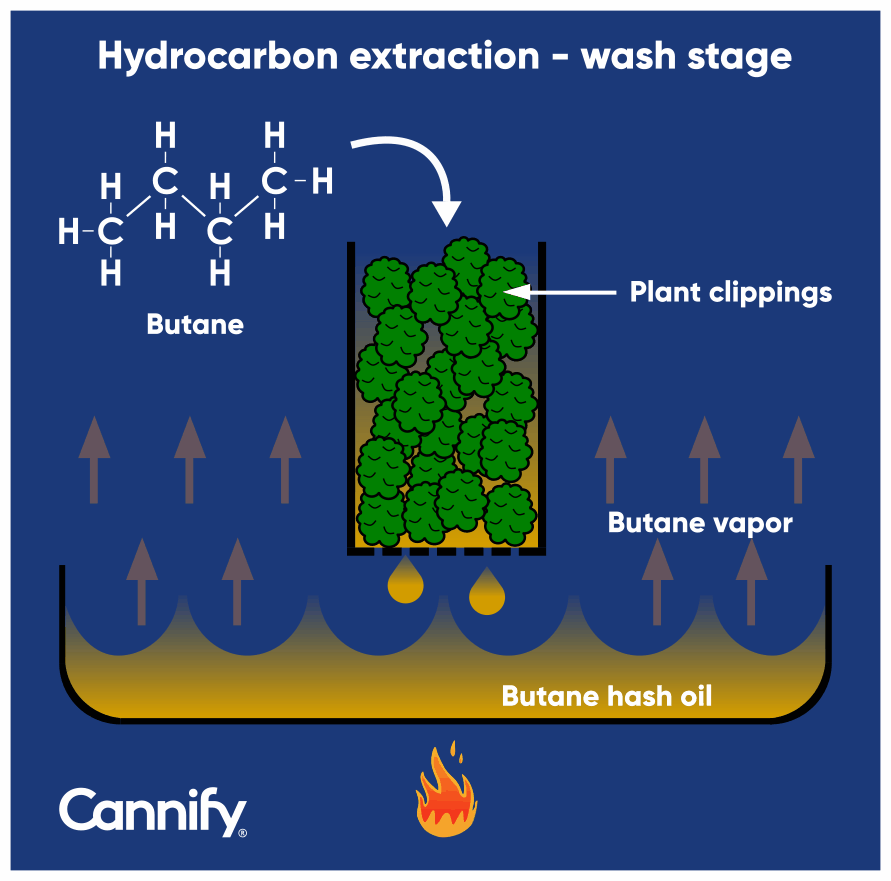 Figure 4: The illustration of the beginning of the hydrocarbon extraction known as was (original illustration by Bell et al., 2015.3).
Figure 4: The illustration of the beginning of the hydrocarbon extraction known as was (original illustration by Bell et al., 2015.3).
Watch a brief summary video on
Cannabis concentrates and extracts made in collaboration with
Enlighten on
YouTube or on
the screens page.
- Raber, Jeffrey C.; Elzinga, Sytze; Kaplan, Charles (2015). Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. The Journal of Toxicological Sciences, 40(6), 797--803.
- Rosenthal, Ed (2014). Beyond Buds: Marijuana ExtractsÑHash, Vaping, Dabbing, Edibles and Medicines. Quick Trading Company.
- Bell, Cameron; Slim, Jessica; Flaten, Hanna K.; Lindberg, Gordon; Arek, Wiktor; Monte, Andrew A. (2015). Butane Hash Oil Burns Associated with Marijuana Liberalization in Colorado. Journal of Medical Toxicology, 11(4), 422-425.
- Al-Zouabi, Ihsan; Stogner, John M.; Miller, Bryan Lee; Lane, Elizabeth S. (2018). Butane hash oil and dabbing: insights into use, amateur production techniques, and potential harm mitigation. Substance Abuse and Rehabilitation, 9, 91-101.
- Harris, D. (2005). Butane encephalopathy. Emergency Medicine Journal, 22(9), 676-677.
- Sen, Ahmet; Erdivanli, Basar (2015). Cardiac arrest following butane inhalation. Anesthesia: Essays and Researches, 9(2), 273.
- Azmir, J.; Zaidul, I. S. M.; Rahman, M. M.; Sharif, K. M.; Mohamed, A.; Sahena, F.; Jahurul, M. H. A.; Ghafoor, K.; Norulaini, N. A. N.; Omar, A. K. M. (2013). Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering, 117(4), 426--436.
 Figure 1: The difference between the contents of cannabis compounds in a raw hemp flower and a hydrocarbon hemp extract (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/)
Figure 1: The difference between the contents of cannabis compounds in a raw hemp flower and a hydrocarbon hemp extract (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/)
 Figure 2: Butane bubbles evaporating before whipping and vacuum purging (image taken from Vjiced, Butane honey oil being evaporated, CC BY-SA 3.0).
Figure 2: Butane bubbles evaporating before whipping and vacuum purging (image taken from Vjiced, Butane honey oil being evaporated, CC BY-SA 3.0).
 Figure 3: MK III Closed loop essential oil extractor (image taken from Emerald Gold Extractors).
Figure 3: MK III Closed loop essential oil extractor (image taken from Emerald Gold Extractors).
 Figure 4: The illustration of the beginning of the hydrocarbon extraction known as was (original illustration by Bell et al., 2015.3).
Watch a brief summary video on Cannabis concentrates and extracts made in collaboration with Enlighten on YouTube or on the screens page.
References:
Figure 4: The illustration of the beginning of the hydrocarbon extraction known as was (original illustration by Bell et al., 2015.3).
Watch a brief summary video on Cannabis concentrates and extracts made in collaboration with Enlighten on YouTube or on the screens page.
References:_logo.svg)