Solvent-based extraction
There are several extraction types based on using different solvents {call-out, text: Chemicals that dissolve solids into liquids.}. The type of solvent influences which and how many compounds are extracted from the compound mixture in cannabis flower. Compounds such as cannabinoids and terpenes are attracted to the solvent based on polarity, which is related to the charge distribution in a molecule. Polar compounds are mostly water-soluble (hydrophilic), and most non-polar compounds are not (hydrophobic). Besides the solvent itself, other factors such as temperature and pressure play a role in your extraction process. You can compare it with extracting coffee or tea: when you make coffee with cold water, it will taste differently than when you make coffee under a higher temperature or different pressure.
When you extract cannabis oil with different solvents, you will yield different ratios of neutral cannabinoids, acid cannabinoids, and terpenes. In Figure 1 you can see an overview of hydrocarbon, CO
2 (carbon dioxide), ethanol (a form of alcohol), and H
2O (water) solvents and the cannabis compounds that will be most efficiently extracted by these solvents. Hydrocarbon extraction will yield more non-polar compounds such as terpenes, than other solvents, whereas ethanol typically yields more cannabinoids. CO
2, supercritical CO
2, or SFE (supercritical fluid extraction) is a more versatile extraction technique, that can change its properties based on the temperature and pressure.
In this section we will cover extractions based on
Water,
Ethanol,
Carbon Dioxide, and
Hydrocarbon.
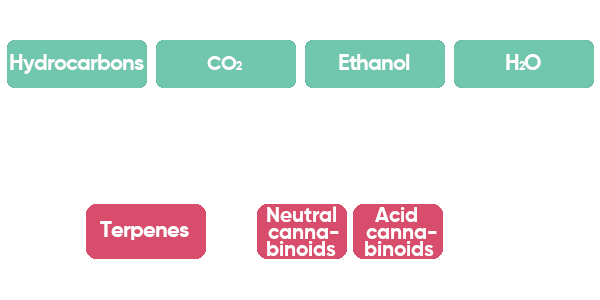 Figure 1: This overview of popular solvents (green boxes) shows them in the order of electric charge (polarity), which influences the molecules that are most efficiently extracted by that solvent (pink boxes). CO2 is carbon dioxide, and H2O is water. Acid cannabinoids are the compounds that are directly made by the plant, such as THCA and CBDA. Neutral cannabinoids are cannabinoids that have lost their acid-group, such as THC and CBD. The difference in acid group changes the effect of the cannabinoid on the body. In the figure, you can see that hydrocarbon extraction is the least polar solvent, whereas water is the most polar solvent. For example, ethanol extraction will yield fewer terpenes than hydrocarbon extraction (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/).
Figure 1: This overview of popular solvents (green boxes) shows them in the order of electric charge (polarity), which influences the molecules that are most efficiently extracted by that solvent (pink boxes). CO2 is carbon dioxide, and H2O is water. Acid cannabinoids are the compounds that are directly made by the plant, such as THCA and CBDA. Neutral cannabinoids are cannabinoids that have lost their acid-group, such as THC and CBD. The difference in acid group changes the effect of the cannabinoid on the body. In the figure, you can see that hydrocarbon extraction is the least polar solvent, whereas water is the most polar solvent. For example, ethanol extraction will yield fewer terpenes than hydrocarbon extraction (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/).
 Figure 2: Laboratory work on plant essences and cannabinoid material (image taken from Draconis44, CCE by Draconis- laboratory work on plant essences and cannabinoid material, CC BY-SA 4.0).
Figure 2: Laboratory work on plant essences and cannabinoid material (image taken from Draconis44, CCE by Draconis- laboratory work on plant essences and cannabinoid material, CC BY-SA 4.0).
Watch a brief summary video on
Cannabis concentrates and extracts made in collaboration with
Enlighten on
YouTube or on
the screens page.
 Figure 1: This overview of popular solvents (green boxes) shows them in the order of electric charge (polarity), which influences the molecules that are most efficiently extracted by that solvent (pink boxes). CO2 is carbon dioxide, and H2O is water. Acid cannabinoids are the compounds that are directly made by the plant, such as THCA and CBDA. Neutral cannabinoids are cannabinoids that have lost their acid-group, such as THC and CBD. The difference in acid group changes the effect of the cannabinoid on the body. In the figure, you can see that hydrocarbon extraction is the least polar solvent, whereas water is the most polar solvent. For example, ethanol extraction will yield fewer terpenes than hydrocarbon extraction (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/).
Figure 1: This overview of popular solvents (green boxes) shows them in the order of electric charge (polarity), which influences the molecules that are most efficiently extracted by that solvent (pink boxes). CO2 is carbon dioxide, and H2O is water. Acid cannabinoids are the compounds that are directly made by the plant, such as THCA and CBDA. Neutral cannabinoids are cannabinoids that have lost their acid-group, such as THC and CBD. The difference in acid group changes the effect of the cannabinoid on the body. In the figure, you can see that hydrocarbon extraction is the least polar solvent, whereas water is the most polar solvent. For example, ethanol extraction will yield fewer terpenes than hydrocarbon extraction (original illustration created by Dr. Markus Roggen, https://www.cbdvl.com/).
 Figure 2: Laboratory work on plant essences and cannabinoid material (image taken from Draconis44, CCE by Draconis- laboratory work on plant essences and cannabinoid material, CC BY-SA 4.0).
Watch a brief summary video on Cannabis concentrates and extracts made in collaboration with Enlighten on YouTube or on the screens page.
Figure 2: Laboratory work on plant essences and cannabinoid material (image taken from Draconis44, CCE by Draconis- laboratory work on plant essences and cannabinoid material, CC BY-SA 4.0).
Watch a brief summary video on Cannabis concentrates and extracts made in collaboration with Enlighten on YouTube or on the screens page.
_logo.svg)